On January 24, 2024, Azurity Pharmaceuticals, Inc., based in Woburn, Massachusetts, has initiated a voluntary recall of a specific lot (F230169A) of Zenzedi® CII (dextroamphetamine sulfate tablets, USP) 30 mg at the consumer level. This recall is prompted by a pharmacist in Nebraska who discovered Carbinoxamine Maleate, an antihistamine drug, in a bottle labeled as Zenzedi® 30 mg tablets. Following this incident, the manufacturer initiated a product complaint and subsequent investigation.
| Zenzedi® CII 30mg Lot Number | F230169A |
| Expiration Date | 2025-06 |
| Suspected Tablet | Carbinoxamine Maleate Tablets USP, 4 mg, white round with GL and 211 embossed on either side |
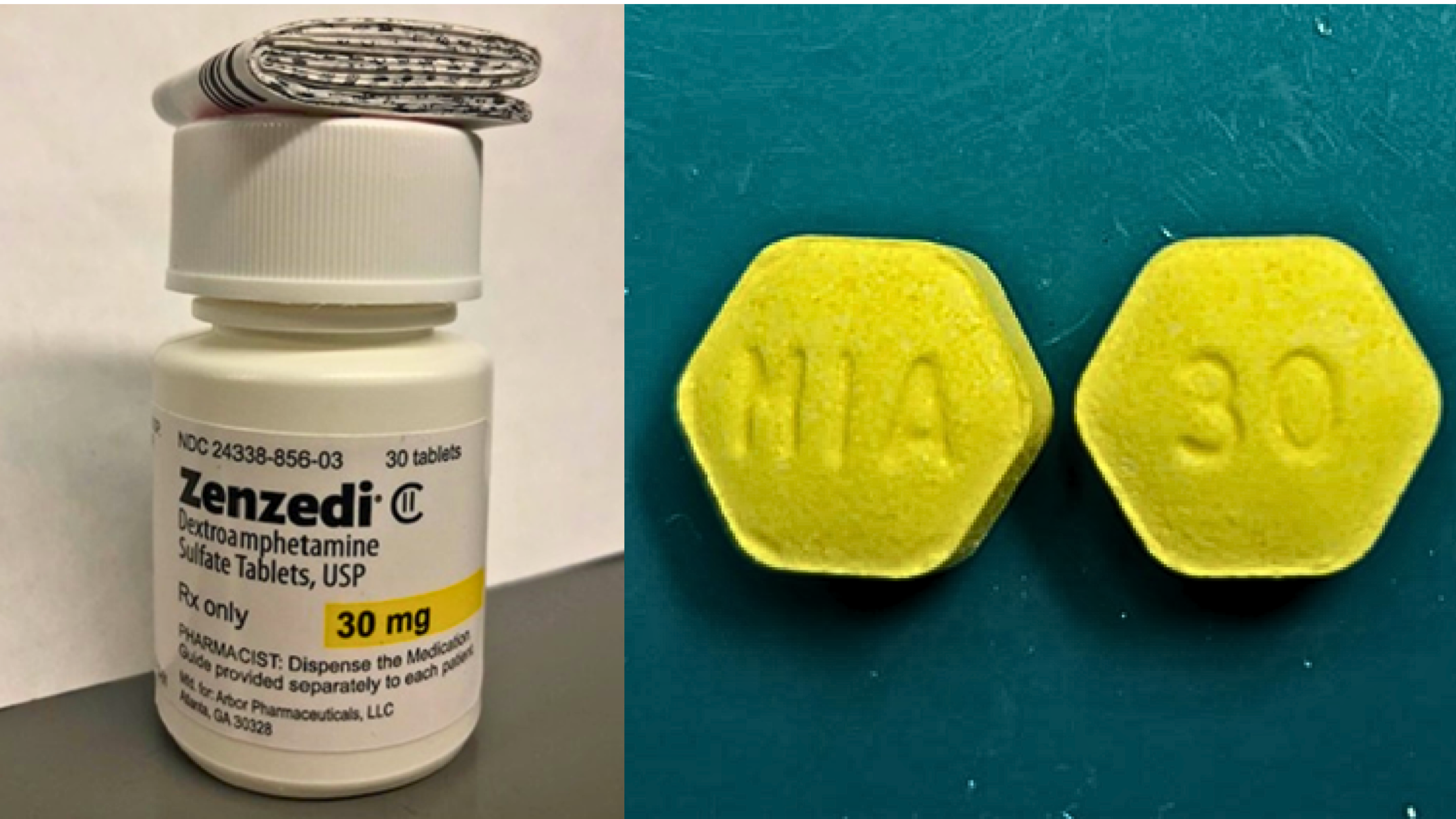
| What Zenzedi 30 mg looks like | Yellow, Hexagonal with MIA and 30 embossed on it on either side |
| Additional safety information | www.azurity.com |
| Report Adverse events to Azurity | aereports@azurity.com |
| Report Adverse events to FDA’s MedWatch Adverse Event Reporting | www.fda.gov/medwatch/report.htm, or calling 1-800-332-1088. |
Patients who inadvertently ingest carbinoxamine instead of Zenzedi® may experience undertreatment of their symptoms, leading to functional impairment and an elevated risk of accidents or injury.
Adverse events associated with carbinoxamine consumption include drowsiness, sleepiness, central nervous system (CNS) depression, increased eye pressure, enlarged prostate urinary obstruction, and thyroid disorder.
For individuals with Attention Deficit Hyperactivity Disorder (ADHD) and Narcolepsy, the sedating effects of carbinoxamine pose a reasonable risk of accidents or injuries, potentially resulting in ongoing disability or, in severe cases, death—especially during activities requiring heightened focus and alertness, such as driving or operating heavy machinery.
As of now, Azurity has not received reports of serious adverse events related to this recall.
Zenzedi® is a prescription medication used for the treatment of Narcolepsy and ADHD. It is marketed under the Arbor Pharmaceuticals, LLC brand, a subsidiary of Azurity Pharmaceuticals, Inc.
The Zenzedi® 30 mg tablets subject to recall are light yellow hexagonal tablets debossed with “30” on one side and “MIA” on the other, packaged in a white bottle with black writing, highlighting “30 mg” in yellow. The suspect tablets (Carbinoxamine Maleate Tablets USP, 4 mg), described by the reporting pharmacist, are white round tablets imprinted with “GL” on one side and “211” on the other. These products were distributed nationwide through pharmacies.
The recall, identified by the NDC No. 24338-856-03, Lot No. F230169A, with an expiration date of 2025-06, covers shipments from August 23, 2023, to November 29, 2023.
Azurity Pharmaceuticals, Inc. sent recall notification letters to wholesale distributors via overnight delivery on January 4, 2024, and has coordinated the return of all recalled products at the wholesaler level. Consumers possessing the recalled product are advised to cease usage and return it to the place of purchase. Azurity is actively collaborating with wholesalers and retailers to facilitate the return and replacement of the affected product, utilizing the services of Inmar Intelligence located at 3845 Grand Lakes Way, Grand Prairie, TX 75050.
For further information on the recall process, contact Inmar Intelligence at 877-804-2069 (Monday through Friday, 9 AM-5 PM EST). Medical or technical inquiries, as well as reporting of technical product complaints or adverse events, can be directed to 800-461-7449 (Monday through Friday, 9 AM-5 PM EST).
Consumers experiencing issues related to the use of this drug product should consult their physician or healthcare provider. Adverse events can also be reported to Azurity via email at aereports@azurity.com.
Adverse reactions or quality problems with this product may be reported to the FDA’s MedWatch Adverse Event Reporting program online at www.fda.gov/medwatch/report.htm, by regular mail or fax using the downloadable form at www.fda.gov/MedWatch/getforms.htm or calling 1-800-332-1088.
U.S. Food and Drug Administration is cooperating with drug recall.
Leave a Reply